Have you ever wondered how chemists represent atoms and their bonding capabilities in a simple yet effective way? The Lewis symbol for nitrogen is a cornerstone of chemical notation, offering insights into the behavior of nitrogen atoms in various compounds. As one of the most abundant elements in the Earth's atmosphere, nitrogen plays a vital role in biological and industrial processes. Understanding its Lewis symbol helps us decode the mysteries of molecular interactions and chemical reactions. In this article, we'll delve deep into the significance of the Lewis symbol for nitrogen, exploring its applications, history, and practical uses.
The Lewis symbol for nitrogen is more than just a representation of an atom; it's a key to unlocking the secrets of chemical bonding. Developed by Gilbert N. Lewis in the early 20th century, this notation system allows scientists to visualize electron configurations and predict how atoms will interact. For nitrogen, the Lewis symbol reveals its five valence electrons, which determine its reactivity and bonding tendencies. This knowledge is crucial for understanding nitrogen's role in everything from ammonia production to DNA synthesis.
As we explore the Lewis symbol for nitrogen, we'll uncover its historical origins, examine its applications in modern chemistry, and discuss its relevance in both theoretical and practical contexts. Whether you're a student, a professional chemist, or simply someone curious about the world of chemistry, this article will provide a comprehensive overview of why the Lewis symbol for nitrogen is so important. So, let's dive in and unravel the fascinating world of chemical notation!
Read also:Why Is The Chicken Big Mac Unavailable A Comprehensive Look At Mcdonalds Menu Mystery
Table of Contents
- 1. What is the Lewis Symbol for Nitrogen?
- 2. Why is the Lewis Symbol Important in Chemistry?
- 3. How is the Lewis Symbol for Nitrogen Used in Chemical Reactions?
- 4. What Are the Historical Roots of the Lewis Symbol?
- 5. Can the Lewis Symbol Predict Nitrogen's Behavior in Compounds?
- 6. Exploring the Applications of the Lewis Symbol in Modern Science
- 7. Common Misconceptions About the Lewis Symbol for Nitrogen
- 8. Frequently Asked Questions About the Lewis Symbol for Nitrogen
What is the Lewis Symbol for Nitrogen?
The Lewis symbol for nitrogen is a visual representation of the atom's valence electrons. In this notation, nitrogen is depicted as "N" surrounded by five dots, each representing one of its valence electrons. These dots are strategically placed around the element symbol to indicate the atom's electron configuration and its potential for bonding. The Lewis symbol for nitrogen is particularly useful because it highlights the element's ability to form triple bonds, which is a defining characteristic of nitrogen's chemistry.
Understanding the Lewis symbol for nitrogen requires a basic grasp of atomic structure. Nitrogen, with an atomic number of 7, has seven electrons in total. Two of these electrons occupy the innermost shell, leaving five in the outermost shell, or valence shell. These five valence electrons are what determine nitrogen's chemical behavior and its ability to form covalent bonds with other atoms. By examining the Lewis symbol, chemists can predict how nitrogen will interact with other elements to form stable compounds.
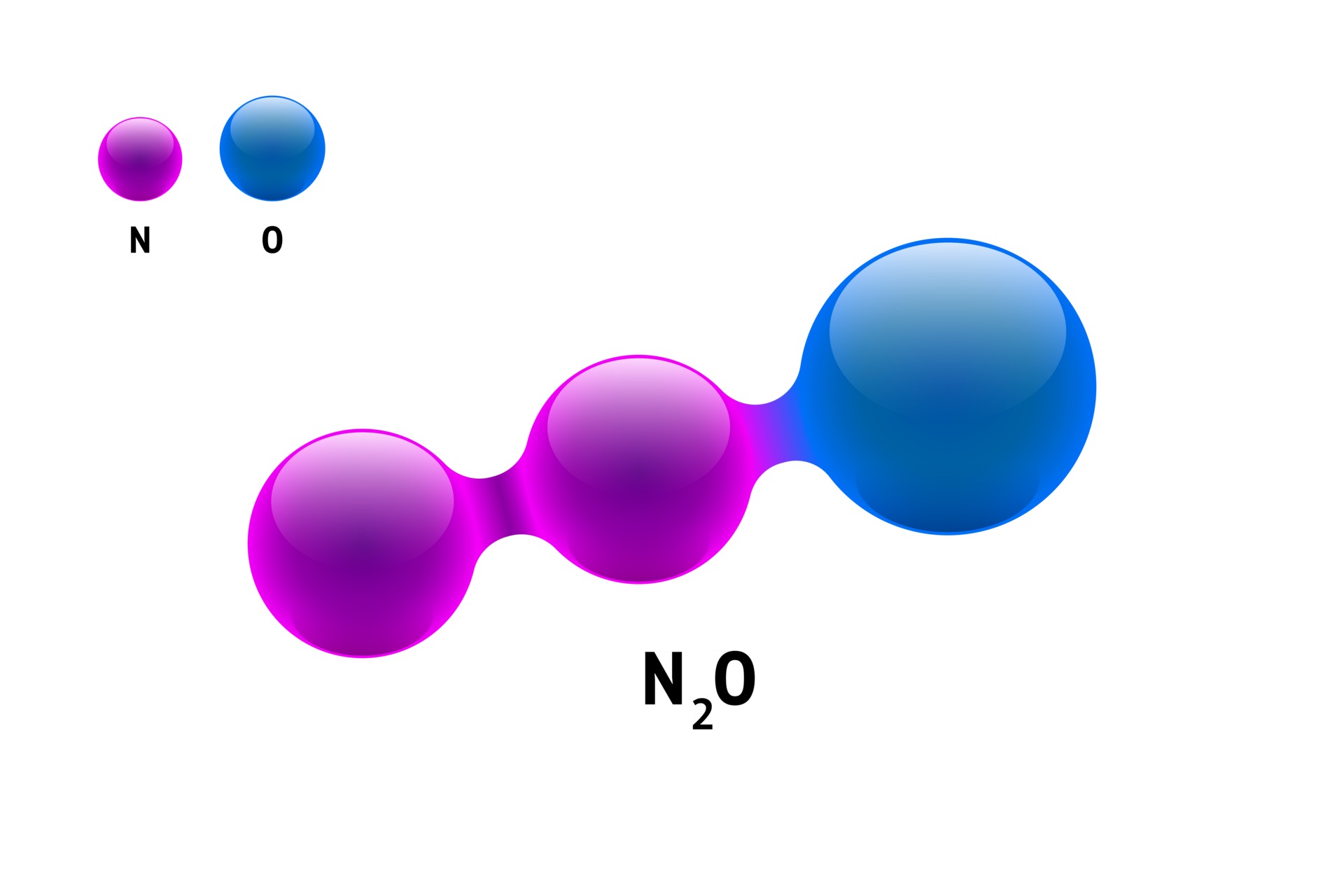
The Lewis symbol for nitrogen is not only a tool for understanding individual atoms but also a foundation for studying molecular structures. For example, in ammonia (NH₃), the Lewis structure shows nitrogen forming three single bonds with hydrogen atoms, leaving one lone pair of electrons. This arrangement explains ammonia's trigonal pyramidal shape and its role as a base in chemical reactions. Similarly, in nitrogen gas (N₂), the Lewis structure reveals a triple bond between two nitrogen atoms, accounting for the molecule's stability and inertness under normal conditions.
Why is the Lewis Symbol Important in Chemistry?
The Lewis symbol is a fundamental concept in chemistry because it simplifies complex atomic interactions into a visual format that is easy to interpret. By focusing on valence electrons, the Lewis symbol allows chemists to predict how atoms will bond and what types of compounds they will form. This is particularly important for nitrogen, which is a versatile element capable of forming a wide range of compounds, from simple molecules like N₂ to complex biomolecules like proteins.
One of the key advantages of the Lewis symbol is its ability to illustrate electron sharing and transfer. In covalent bonds, electrons are shared between atoms to achieve a stable electron configuration, often resembling that of noble gases. The Lewis symbol for nitrogen clearly shows its five valence electrons, making it evident that nitrogen needs three additional electrons to achieve a stable configuration. This insight is crucial for understanding nitrogen's tendency to form triple bonds, as seen in N₂, or single bonds, as seen in ammonia.
Beyond its predictive power, the Lewis symbol also serves as a teaching tool. For students learning chemistry, it provides a tangible way to visualize abstract concepts like electron configurations and bonding. By mastering the Lewis symbol for nitrogen, students can build a strong foundation for more advanced topics, such as molecular geometry, resonance structures, and reaction mechanisms.
Read also:How To Craft The Perfect Felicitaciones De Graduacioacuten De Universidad A Comprehensive Guide
How is the Lewis Symbol for Nitrogen Used in Chemical Reactions?
Chemical reactions involving nitrogen are often governed by the principles of electron sharing and transfer, which are clearly illustrated by the Lewis symbol for nitrogen. For instance, in the Haber-Bosch process, nitrogen gas (N₂) reacts with hydrogen gas (H₂) to produce ammonia (NH₃). The Lewis structure of N₂ shows a triple bond between the two nitrogen atoms, which must be broken for the reaction to proceed. This requires a significant amount of energy, making the Haber-Bosch process both energy-intensive and economically significant.
In biological systems, nitrogen's Lewis symbol helps explain its role in amino acids and nucleic acids. Amino acids, the building blocks of proteins, contain nitrogen atoms that participate in peptide bond formation. The Lewis structure of nitrogen in these molecules reveals how it forms single bonds with carbon and hydrogen atoms, contributing to the overall stability of the protein structure. Similarly, in DNA and RNA, nitrogenous bases like adenine and guanine rely on nitrogen's ability to form specific hydrogen bonds, ensuring the accuracy of genetic information transfer.
Industrial applications of nitrogen also benefit from an understanding of its Lewis symbol. For example, in the production of nitric acid (HNO₃), nitrogen undergoes a series of oxidation states, each of which can be represented by a Lewis structure. These structures help chemists optimize reaction conditions and improve yield, making nitric acid one of the most widely used chemicals in the world.
What Are the Historical Roots of the Lewis Symbol?
The origins of the Lewis symbol can be traced back to the early 20th century, when American chemist Gilbert N. Lewis introduced his theory of electron-pair bonding. In 1916, Lewis published a groundbreaking paper titled "The Atom and the Molecule," in which he proposed that chemical bonds are formed through the sharing of electron pairs. This concept laid the foundation for modern valence bond theory and introduced the dot notation that we now recognize as the Lewis symbol.
At the time, Lewis's ideas were revolutionary, as they provided a clear explanation for the behavior of atoms in molecules. By focusing on valence electrons, Lewis was able to predict the bonding patterns of many elements, including nitrogen. His work was later expanded upon by other chemists, such as Linus Pauling, who developed the concept of resonance and further refined the understanding of molecular structures.
Today, the Lewis symbol remains a cornerstone of chemical education and research. Its simplicity and effectiveness have ensured its continued relevance in both theoretical and applied chemistry. For nitrogen, the Lewis symbol has proven to be an invaluable tool for understanding its role in everything from atmospheric chemistry to biological processes.
Can the Lewis Symbol Predict Nitrogen's Behavior in Compounds?
Yes, the Lewis symbol for nitrogen is a powerful predictor of its behavior in compounds. By examining the arrangement of valence electrons, chemists can anticipate how nitrogen will interact with other elements and what types of bonds it will form. For example, in nitrogen oxides like NO₂, the Lewis structure reveals a double bond between nitrogen and oxygen, with one additional lone pair on the nitrogen atom. This arrangement explains the molecule's reactivity and its role as a precursor to acid rain.
In organic chemistry, nitrogen's Lewis symbol helps explain its behavior in functional groups like amines and amides. Amines, which contain nitrogen atoms bonded to carbon and hydrogen, often exhibit basic properties due to the presence of a lone pair of electrons on nitrogen. This lone pair can accept protons, making amines useful as bases in various reactions. Similarly, in amides, nitrogen forms single bonds with carbon and hydrogen, contributing to the molecule's stability and resistance to hydrolysis.
Exploring the Applications of the Lewis Symbol in Modern Science
In modern science, the Lewis symbol for nitrogen finds applications in a wide range of fields, from materials science to environmental chemistry. For example, in the development of advanced materials, nitrogen's Lewis symbol helps predict the properties of compounds like nitrides and azides. These materials are used in everything from electronics to aerospace engineering, where their unique properties make them indispensable.
In environmental science, the Lewis symbol for nitrogen is crucial for understanding atmospheric processes. Nitrogen oxides, formed through combustion processes, contribute to air pollution and climate change. By studying their Lewis structures, scientists can develop strategies to mitigate their effects and improve air quality. Similarly, in agricultural science, the Lewis symbol for nitrogen aids in the optimization of fertilizers, ensuring that plants receive the right balance of nutrients for healthy growth.
Medical research also benefits from the insights provided by the Lewis symbol for nitrogen. In drug design, nitrogen-containing compounds like amines and amides are common due to their ability to interact with biological targets. Understanding the Lewis structures of these molecules helps chemists design drugs with improved efficacy and reduced side effects, advancing the field of medicinal chemistry.
Common Misconceptions About the Lewis Symbol for Nitrogen
Despite its widespread use, the Lewis symbol for nitrogen is often misunderstood by students and non-chemists alike. One common misconception is that the Lewis symbol represents the actual arrangement of electrons in an atom. In reality, the Lewis symbol is a simplified model that focuses on valence electrons and their potential for bonding. It does not account for the complex quantum mechanical behavior of electrons in an atom.
Another misconception is that the Lewis symbol can always predict the exact structure of a molecule. While it is a powerful tool for understanding bonding patterns, the Lewis symbol does not account for factors like molecular geometry and resonance. For example, in molecules with multiple resonance structures, the Lewis symbol alone cannot determine the most stable configuration. In such cases, additional concepts like molecular orbital theory must be applied.
Finally, some people believe that the Lewis symbol is outdated and no longer relevant in modern chemistry. However, this is far from the truth. The Lewis symbol remains a fundamental concept in chemical education and research, providing a foundation for more advanced theories and applications. Its simplicity and effectiveness ensure its continued use in both theoretical and practical contexts.
What Are Some Frequently Asked Questions About the Lewis Symbol for Nitrogen?
FAQ 1: How Many Valence Electrons Does Nitrogen Have?
Nitrogen has five valence electrons. These electrons are represented in the Lewis symbol for nitrogen as five dots surrounding the element symbol "N."
FAQ 2: Can Nitrogen Form Single, Double, and Triple Bonds?
Yes, nitrogen can form single, double, and triple bonds depending on the molecule and its bonding partners. In N₂, nitrogen forms a triple bond, while in ammonia (NH₃), it forms three single bonds with hydrogen atoms.
FAQ 3: Why Is the Lewis Symbol Important for Understanding Nitrogen's Chemistry?
The Lewis symbol for nitrogen is important because it provides a visual representation of the atom's valence electrons and its potential for bonding. This knowledge is crucial for predicting how nitrogen will interact with other elements to form stable compounds.
Conclusion
The Lewis symbol for nitrogen is a cornerstone of chemical notation, offering insights into the behavior of one of the most important elements in the periodic table. From its historical origins to its modern applications, the Lewis symbol continues to play a vital role in chemistry education and research. By understanding the Lewis symbol for nitrogen, we can unlock the secrets of molecular interactions and gain a deeper appreciation for the fascinating world of chemistry.
In conclusion, the Lewis symbol for nitrogen is more than just a representation of an atom; it's a key to understanding the fundamental principles of chemical bonding. Whether you're studying nitrogen's role in biological processes, industrial applications, or environmental chemistry, the Lewis symbol provides a foundation for exploring the complexities of molecular structures and reactions. So, the next time you encounter the Lewis symbol for nitrogen, remember its significance and the wealth of knowledge it represents!